Government of India had mandated thru its (GSR20(E)) to implement a QR Code based barcode label print for all Active Pharmaceutical Ingredients (APIs) manufactured OR imported to India. This mandate goes live on Jan 1, 2023.
The mandate has been brought in as an effort to combat the issue of counterfeit medicines in India and to serialize the API’s at the source for manufacturing or import before they are consumed in formulation of drug. This QR Code will also help in maintain the Genealogy of the product for backward traceability of its ingredients
QR Code Data Points
The QR Code Labels contains the following data points
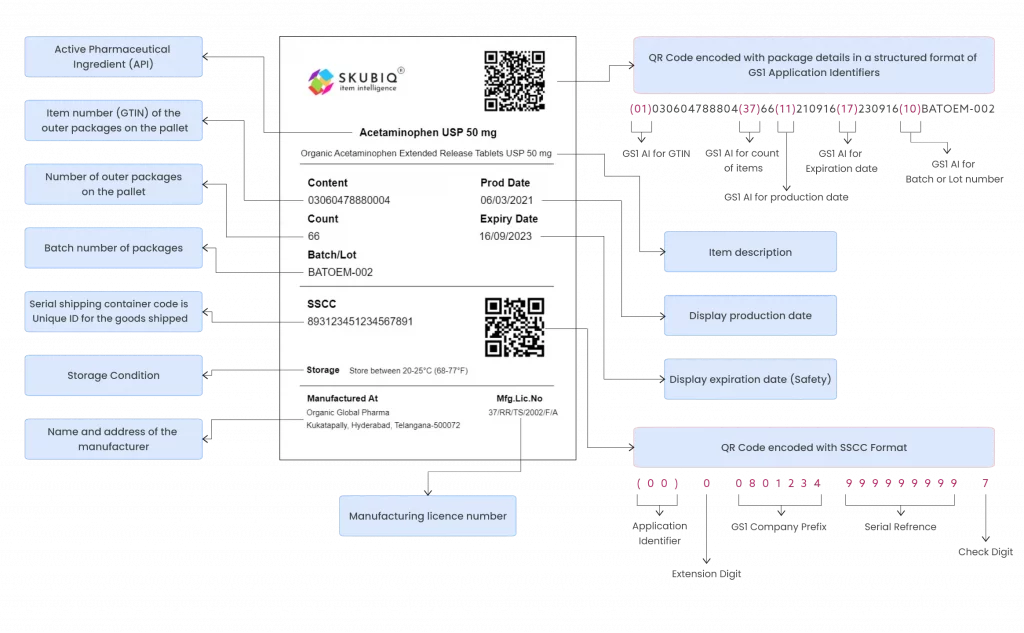
- Unique product identification code (GS1 Based)
- Name of the API
- Brand name (if any)
- Name and address of the manufacturer
- Batch number
- Batch size
- Date of manufacturing
- Date of expiry or retesting
- Serial shipping container code (SSCC)
- Manufacturing license number or import license number
- Special storage conditions required (if any)
GS1 -Sample QR Code
How SKUBIQ can help you in building the QR code label and maintain the historical data for backward traceability.
SKUBIQ , seamlessly integrates with your standard Line-of-Business applications to sync the Master and Transactional data using the Secure OpenAPI’s .
GS1 QR Code will be generated and the required material storage data points such as Job Order, Batch/Lot, Mfg.Date Exp.Date, Temperature condition data will be maintained in the SKUBIQ with a 21 CFR Part 11 compliance requirements and SSCC Code will be posted to EPCIS.
With an 8 years archived storage of REST historical data in SKUBIQ , you can trace the material flow from the archives and check for control of counterfeit drugs. SKUBIQ can be used to tis full potential on the production lines to help maintain the genealogy of the bulk API’s and the formulations .



